Understanding the Statement of Deficiencies and Plan of Correction (CMS-2567)
Introduction
The Statement of Deficiencies and Plan of Correction, commonly referred to as CMS-2567, is a critical document in the healthcare industry. It is used by the Centers for Medicare & Medicaid Services (CMS) to communicate with healthcare providers about deficiencies found during surveys or inspections. Understanding and properly addressing this document is vital for maintaining compliance and ensuring high-quality patient care.
What is CMS-2567?
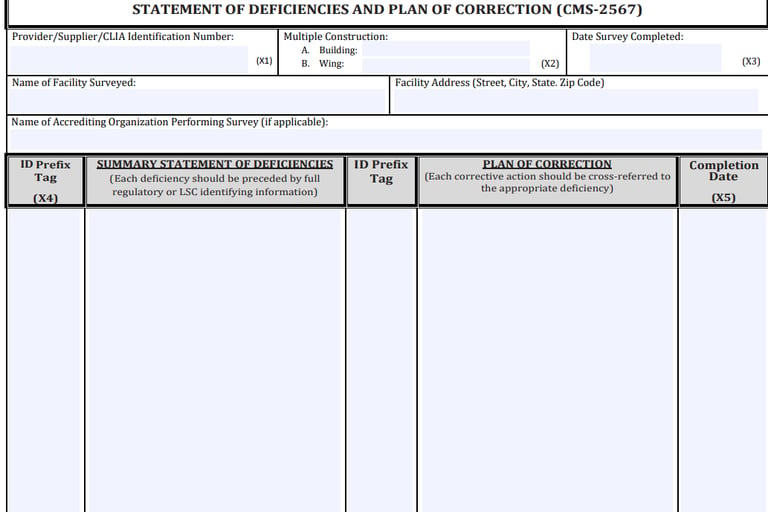
CMS-2567 is a form used by state survey agencies and accrediting organizations to notify healthcare facilities of deficiencies that need correction to meet the standards set by CMS. The form lists specific areas where the facility does not comply with federal health, safety, or quality standards. Each deficiency is detailed with references to the specific regulation violated.
The Importance of CMS-2567
The CMS-2567 form is not just a bureaucratic requirement; it plays a pivotal role in the continuous improvement of healthcare quality. It helps facilities identify and correct gaps in their operations, ultimately leading to better patient outcomes. Additionally, addressing deficiencies promptly and effectively can prevent penalties, including fines or loss of Medicare and Medicaid funding.
Components of CMS-2567
Deficiencies: This section outlines each deficiency found during the survey. It provides a detailed description and the regulatory basis for the finding.
Plan of Correction: For each deficiency, the healthcare facility must outline a Plan of Correction (PoC). This plan should detail the steps the facility will take to correct the deficiency, the responsible person or department, and the timeline for implementation.
Implementation and Monitoring: After submitting the PoC, the facility must implement the corrections within the stipulated time frame. Ongoing monitoring and documentation are necessary to ensure compliance and prevent recurrence of the deficiencies.
Responding to CMS-2567
Immediate Review: Upon receiving CMS-2567, the facility's leadership and compliance team should review each deficiency carefully.
Developing a Plan of Correction: Engage with relevant departments to formulate actionable and measurable steps to address each identified issue. Include specific timelines and responsible individuals.
Submission and Approval: Submit the PoC to the appropriate survey agency or accrediting body. The plan may be subject to approval or further discussion.
Implementation and Documentation: Execute the PoC according to the outlined timeline. Keep detailed records of actions taken and their outcomes.
Preparation for Re-Survey: Often, a follow-up survey is conducted to verify the implementation of the PoC. Facilities should be prepared for this re-survey by ensuring all corrective actions are fully integrated into their operations.
Conclusion
The CMS-2567 form is a tool for transparency and improvement in healthcare facilities. By effectively addressing the deficiencies listed and implementing a robust Plan of Correction, facilities not only comply with regulations but also enhance the quality of care they provide. For healthcare administrators, understanding and navigating the CMS-2567 process is essential for maintaining operational excellence and patient safety.